|
- Home
- BioZoom
- BioZoom archives
- 2018
- 2018 Issue 4
- Therapeutic Potential of CRISPR Mediated Genome Editing
Therapeutic Potential of CRISPR Mediated Genome Editing |
Authors: Henriette Frederiksen, research assistant, and Kristine Freude, associate professor, Department of Veterinary Clinical and Animal Sciences, Faculty of Health and Medical Sciences. University of Copenhagen, henriette.r.frederiksen@sund.ku.dk and kkf@sund.ku.dk. |
It is envisioned that genetic mutations can be corrected through specifically designed CRISPR-Cas9 systems and thereby potentially prevent or cure many diseases. This article presents a general overview of some of the application areas for CRISPR-Cas9 in the development of disease treatment now and in the future. The human genome encodes the blueprint for all cells and structures making up individual organisms. If this genetic carrier of information (DNA) is damaged, mutations can arise affecting the function of specific tissues and parts of the body resulting in various diseases. More than 6000 different diseases are caused by mutations in a single gene, affecting millions of people worldwide. These mutations can be inherited but can also appear later in life as a result of spontaneous DNA damage. Some genetic disorders are more complex, and mutations affect multiple genes. Examples of such diseases include dementia, cancer, heart disease, and infertility, all of which are known to be genetic, but the inheritance pattern and how environmental factors are involved remain unknown. 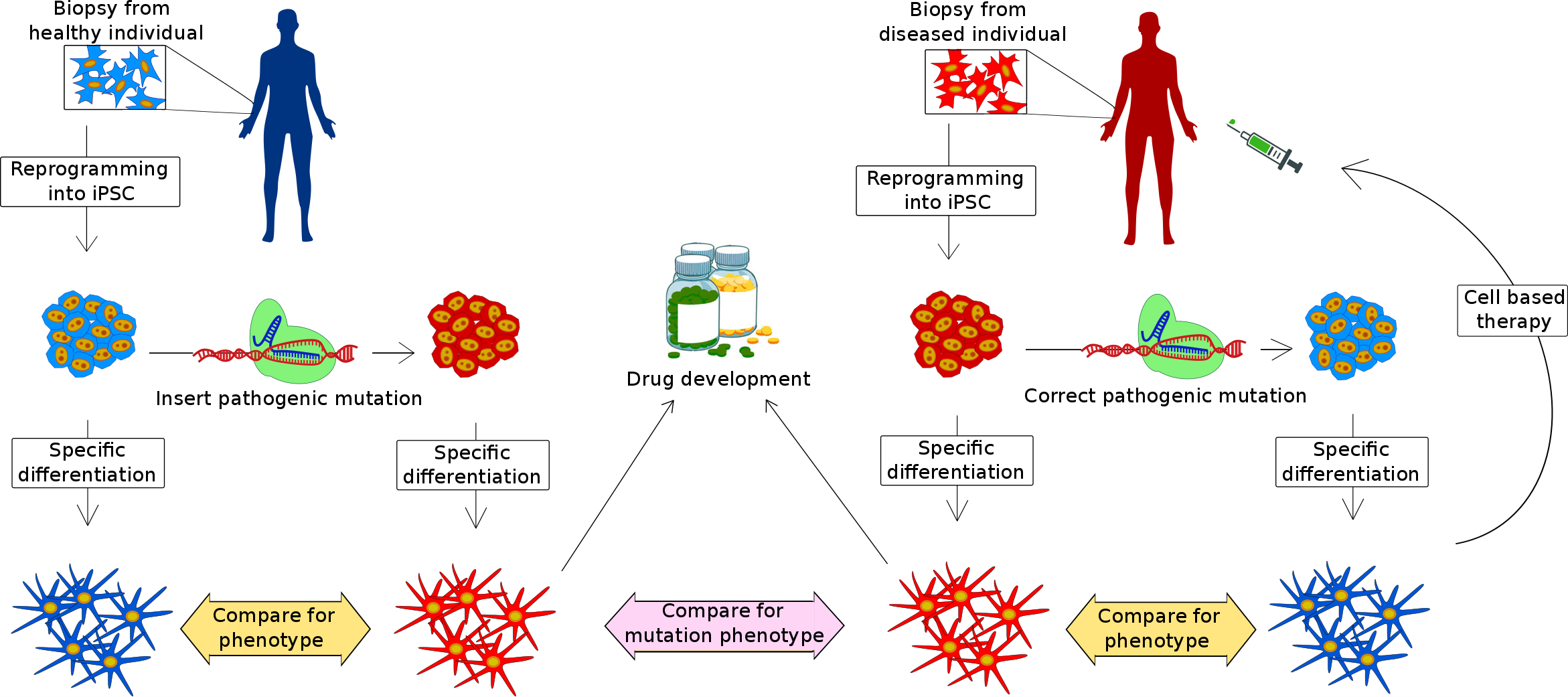 Figure 1: Gene editing using CRISPR-Cas9: A) The 20nt sequence of the crRNA (yellow) attaches to the complimentary genomic DNA (pink). The PAM-site is marked in green and the trans-activating crRNA (tracrRNA) in red. Black arrows show the cleavage site. B) Two pathways for DNA repair: To the left the non-homologous end-joining pathway (NHEJ). The blunt ends of the DNA are stabilized and ‘glued’ back together. In this process indel mutations are common, which results in deactivation of the gene. The path to the right is homology directed reapir (HDR). In this pathway a repair template is required. As the genomic DNA is not stabilized, it will form single stranded ends, that can match with its complimentary sequence on the repair template. The missing part is transcribed and the DNA hereby repaired in a way that allows for insertion of additional genetic material. Figure 1: Gene editing using CRISPR-Cas9: A) The 20nt sequence of the crRNA (yellow) attaches to the complimentary genomic DNA (pink). The PAM-site is marked in green and the trans-activating crRNA (tracrRNA) in red. Black arrows show the cleavage site. B) Two pathways for DNA repair: To the left the non-homologous end-joining pathway (NHEJ). The blunt ends of the DNA are stabilized and ‘glued’ back together. In this process indel mutations are common, which results in deactivation of the gene. The path to the right is homology directed reapir (HDR). In this pathway a repair template is required. As the genomic DNA is not stabilized, it will form single stranded ends, that can match with its complimentary sequence on the repair template. The missing part is transcribed and the DNA hereby repaired in a way that allows for insertion of additional genetic material.
A potential for CRISPR-Cas9-based therapy As CRISPR-Cas9 technology is still young, most of the progress has been made using this technique in early embryos. An American research group has used the CRISPR-Cas9 technique to successfully correct a genetic mutation in human pre-implantation embryos (4). Even though this is a fascinating proof of principle-study, the risk of so-called off target effects does not justify such manipulations in pre-implantation embryos. Off-target effects are Cas9 mediated cuts besides the ones desired in the experiment or treatment. In most cases, it is still preferable to screen embryos before implantation, if a family history of disease exists and subsequently only select and implant mutation free embryos. 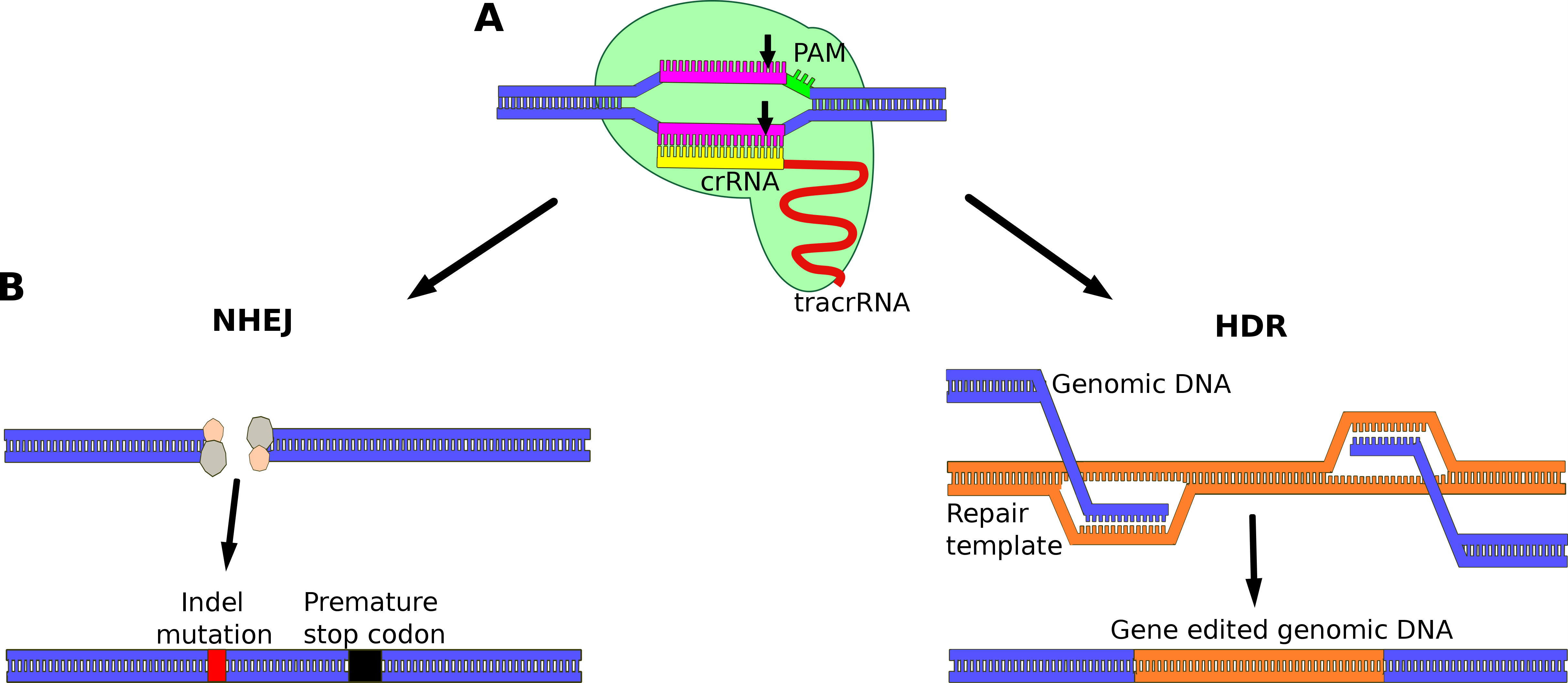 Figure 2: Cell model for study of genetic diseases. Biopsies are obtained from either a healthy individual (blue) or a patient carrying a pathogenic mutation (red). Cells can then be reprogrammed into induced pluripotent stem cells (iPSC) and differentiated into a specific cell type to investigate the cell type specific pathology of the disease. CRISPR-Cas9 can be applied to either correct the mutation in cells from patients or introduce a specific mutation in cells obtained from healthy individuals. Genetically corrected cells can be used for cell based therapy and cells with pathogenic mutations can be used for screening of compounds for drug development. Figure 2: Cell model for study of genetic diseases. Biopsies are obtained from either a healthy individual (blue) or a patient carrying a pathogenic mutation (red). Cells can then be reprogrammed into induced pluripotent stem cells (iPSC) and differentiated into a specific cell type to investigate the cell type specific pathology of the disease. CRISPR-Cas9 can be applied to either correct the mutation in cells from patients or introduce a specific mutation in cells obtained from healthy individuals. Genetically corrected cells can be used for cell based therapy and cells with pathogenic mutations can be used for screening of compounds for drug development.
Even though CRISPR-Cas9 is not used to correct mutations in embryos, the CRISPR-Cas9 system is used in many other ways to treat diseases. American researchers and companies are currently working together to launch a new CRISPR-Cas9-based form of gene-therapy treatment against β-thalassemia and sickle cell disease (5). In their approach, CRISPR-Cas9 is applied for editing of patients’ own hematopoietic stem cells in order to increase their production of fetal hemoglobin. This strategy aims at reducing the need of blood transfusion. Such pilot projects might pave the way to develop similar treatments for other diseases, and could help elucidate potential problems related to the CRISPR-Cas9 mediated editing approach. In principle one can envision that mutated cells obtained from patients can be corrected via CRISPR-Cas9 and transplanted back into the patient. Whilst this is a suitable approach for some diseases such as inherited retinal disorders(6), it might be more challenging for other disease such as Alzheimer’s disease (AD). Neurons are postmitotic cells and replacing such neurons with the purpose of restoring connectivity to existing neurons in an AD patient will be very challenging. Additionally, these neurons or neural progenitors will be placed in an adverse neurotoxic environment impacting their survival and integration capacity (7). CRISPR-Cas9 is a valuable research tool At the moment, CRISPR-Cas9 is mostly used for disease modeling (Figure 2). The advantage of CRISPR-Cas9 is that it allows for the generation of isogenic cell lines, which allow direct comparisons to the parental mutant line. Thereby, the focus is placed on the mutation and genetic background noise from age and gender matched controls can be excluded. Moreover, introduction of disease causing mutations into cell lines obtained from a healthy individual is under less legal restriction, and the wildtype cells can be used to study multiple genetic mutations by insertion. This in addition provides the benefit to create mutant cell lines in sufficient numbers from rare diseases where access to patient material is limited. In our laboratory we study specifically neurodegenerative diseases such as frontotemporal dementia (FTD) and AD. Via Crispr-Cas9 mediated mutation repair we were able to create isogenic lines for our patient iPSC. These were differentiated into neurons and used for comparative studies. The advantage of such disease modeling is that one can conclude that the observed phenotypes are linked to the mutation, if they are absent in the isogenic controls (8).These advanced cell models can potentially help to develop new drugs for treatment and also give a more profound knowledge of some of the molecular mechanism involved in genetic disease(9). Furthermore, CRISPR-Cas9 is used to make genetic screens to identify parts of the DNA that – when knocked out – will ablate abnormal cancerous cells and to understand the influence of different genes in pathological mechanisms (10). Knowledge about cancer cells has led to clinical use of CRISPR-Cas9 to alter T-cells of patients in order to enhance their capability to target cancer cells and hereby destroy them (11). Other groups use CRISPR-Cas9 to increase the susceptibility of bacteria to antibiotic treatment by making cuts in specific parts of the DNA of the bacteria (12). The importance of CRISPR-Cas9 in disease treatment is evident from the broad range of research that uses CRISPR-Cas9 as the key component. CRISPR-Cas9 holds a lot of potential for treatment of various diseases but further assessment of the off-target effects is necessary, before it can be used as more widely applied treatment. Furthermore, the efficiency of CRISPR-Cas9 in gene editing is still quite low, compared to its efficiency to knock out genes. Once these problems are solved, CRISPR-Cas9 can potentially change the way we think about treatment and diseases. References 1. Mojica FJM, Diez-Villaseñor C, Garcia-Martinez J, Soria E. Intervening Sequences of Regularly Spaced Prokaryotic Repeats Derive from Foreign Genetic Elements. J Mol Evol. 2005;60(2):174-182. doi:10.1007/s00239-004-0046-3. 2. Cong L, Ran FA, Cox D, et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science (80- ). 2013;339(6121). 3. Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science (80- ). 2012;337(6096):816-821. doi:10.1126/science.1225829. 4. Ma H, Marti-Gutierrez N, Park S-W, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548(7668):413-419. doi:10.1038/nature23305. 5. CRISPR Therapeutics. http://crisprtx.com/index.php. Accessed September 27, 2018. 6. Burnight ER, Gupta M, Wiley LA, et al. Using CRISPR-Cas9 to Generate Gene-Corrected Autologous iPSCs for the Treatment of Inherited Retinal Degeneration. Mol Ther. 2017;25(9):1999-2013. doi:10.1016/j.ymthe.2017.05.015. 7. Duncan T, Valenzuela M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8. doi:10.1186/S13287-017-0567-5. 8. Zhang Y, Schmid B, Nikolaisen NK, et al. Patient iPSC-Derived Neurons for Disease Modeling of Frontotemporal Dementia with Mutation in CHMP2B. Stem cell reports. 2017;8(3):648-658. doi:10.1016/j.stemcr.2017.01.012. 9. Musunuru K. Genome editing of human pluripotent stem cells to generate human cellular disease models. Dis Model Mech. 2013;6(4):896-904. doi:10.1242/dmm.012054. 10. Chen S, Sanjana NE, Zheng K, et al. Genome-wide CRISPR Screen in a Mouse Model of Tumor Growth and Metastasis. Cell. 2015;160(6):1246-1260. doi:10.1016/j.cell.2015.02.038. 11. Reardon S. First CRISPR clinical trial gets green light from US panel. Nature. June 2016. doi:10.1038/nature.2016.20137. 12. Otoupal PB, Cordell WT, Bachu V, Sitton MJ, Chatterjee A. Multiplexed deactivated CRISPR-Cas9 gene expression perturbations deter bacterial adaptation by inducing negative epistasis. Commun Biol. 2018;1(1):129. doi:10.1038/s42003-018-0135-2. |
Looking for something? Search the website here: |
